Josh Disbrow is chairman and chief executive officer of Aytu BioPharma, which makes a competing drug to treat ADHD called Adzenys XR-ODT.
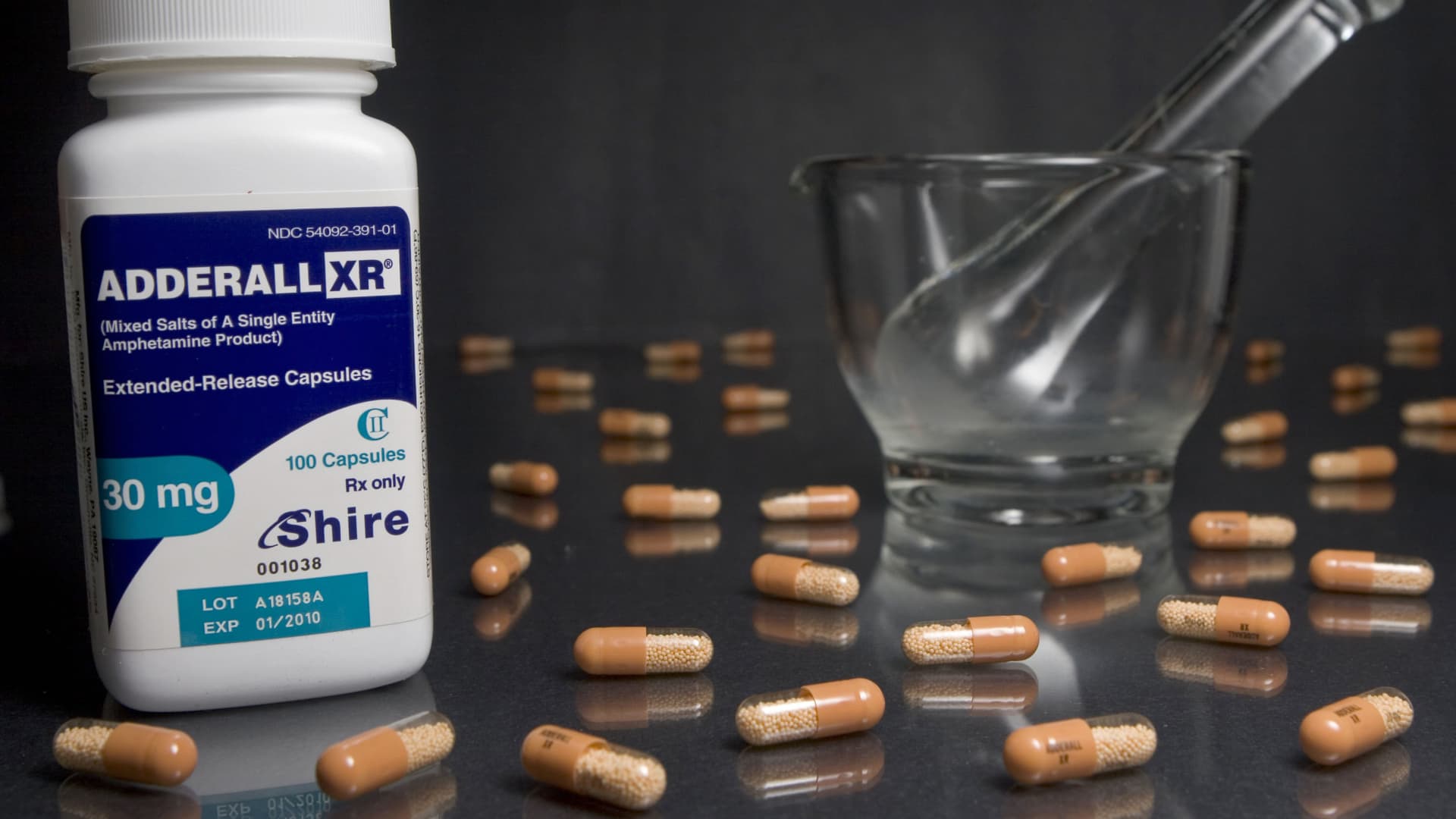
A nationwide shortage of Adderall has left many patients struggling to manage their attention deficit hyperactivity disorder, better known as ADHD. From all indications, supplies are likely to remain scarce for several more months.
The blame for these shortages has been directed at manufacturers, the rise of telehealth medicine, and an increase in prescriptions for and abuse of Adderall. But finger-pointing does little to help the many who depend on consistent access to ADHD medications. Real solutions are in order.
Given the growing prevalence of ADHD among patients of all ages, it’s time for policymakers to rethink how these essential medicines — and the providers who prescribe and dispense them to patients — are regulated. Only then can we ensure that shortages like this one don’t happen again.
ADHD diagnoses have been increasing considerably in recent decades. Between 1999 and 2010, for instance, the prevalence of diagnosed cases among adults rose nearly fivefold. Between 2010 and 2017, diagnoses among children grew by 31%.
As one might imagine, these trends have vastly increased demand for the stimulant medications used to treat ADHD, including Adderall. Among adults aged 22 to 44, in fact, Adderall prescriptions increased by 7.4% between 2019 and 2020. The following year, prescriptions in this age group leapt by more than 15%.
There’s no denying that skyrocketing demand for Adderall combined with manufacturing delays at Teva, one of the drug’s biggest producers, are partly responsible for the current shortage. But supply chains for Adderall wouldn’t be nearly so fragile were it not for some of the federal regulations governing the drug.
Consider the Drug Enforcement Administration’s role in managing the overall availability of ADHD medicines. Because Adderall is designated a Schedule II controlled substance, the DEA imposes aggregate production quotas limiting how much manufacturers can make each year.
In order to set such quotas, the DEA must estimate how much Adderall American patients will require the following year. Should the agency initially underestimate demand and fail to increase its quota in a timely manner in response to new information, the result can be widespread shortages. In 2011, for instance, the pharmaceutical firm Shire pointed to the DEA’s sluggish process for approving quota increases as the underlying cause of that year’s shortage of Adderall XR, a related ADHD medication.
Yet even today, as Adderall continues in short supply around the country, the DEA shows no sign of increasing its production quota anytime soon.
This isn’t the only way in which cumbersome red tape makes it difficult for ADHD patients to remain current on their medications. Food and Drug Administration regulations regarding the substitutability of drugs are another culprit.
In the event a pharmacy runs out of a medicine like Adderall, ideally a pharmacist should be free to substitute a comparable medicine. But under current FDA regulations, only drugs with so-called A or B equivalence codes can be interchanged in this way.
For medicines that don’t meet this strict standard, pharmacists must contact the prescribing doctor, secure a new prescription, and cancel the previous one before dispensing the substitute.
This sort of process is necessary to limit which medicines a pharmacist can dispense without first consulting a patient’s physician. But it can create significant barriers for patients seeking a timely refill. In some cases, the process of substituting for a drug like Adderall can drag on for days, forcing patients to go without — sometimes even to develop painful and disruptive withdrawal symptoms.
The FDA should explore ways to safely provide more substitution flexibility for pharmacists within the current framework, particularly in times of shortages. The agency can also bolster general education for pharmacists and doctors on which comparable medications they can swap.
Updating federal regulations could help prevent the worst consequences of prolonged drug shortages. It’s long past time to do so.